Enhancement of patient compliance and greater control of drug release rates in vivo have been the driving force behind the advancement of novel oral and transdermal drug delivery systems. The ability to predict the bioavailability of a drug in vivo is a necessary condition for any successful drug delivery system. Methods to create drug delivery systems that rapidly disintegrate or delay the release of drug are of particular interest because they can result in situations where patient compliance is increased due to the ease of disintegration or the alleviation of additional medication due to the controlled release. Furthermore, the final drug morphology of the API can be utilized for increased solubility (amorphous form) or increased stability and storage (crystalline form) (Pyo 2007).
The goal of this research is to utilize Drop-on-Demand technology to manufacture innovative drug delivery systems utilizing a systematic approach. Deposition of drug solution via Drop on Demand (DoD) technology can be used to predict and manipulate final drug structure resulting in control of the disintegration of the drug delivery system and drug dissolution. Deposition pattern and final drug structure has been found to impact dissolution.
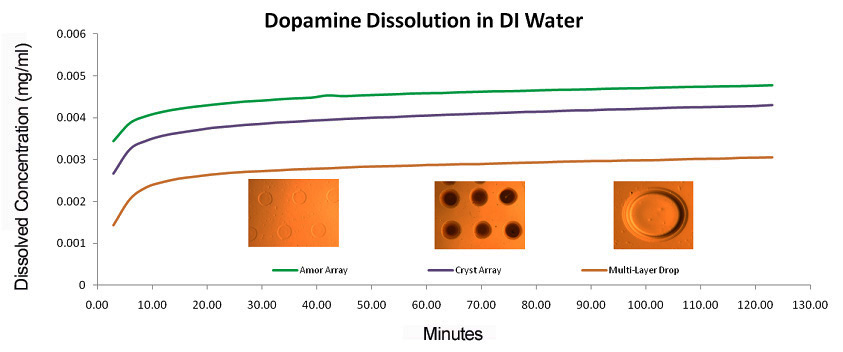
Figure 1. Dopamine dissolution rate for printed dopamine droplets dissolved in water (a) Amorphous Array, (b) Crystalline Array, (c) Multilayer Droplet.
